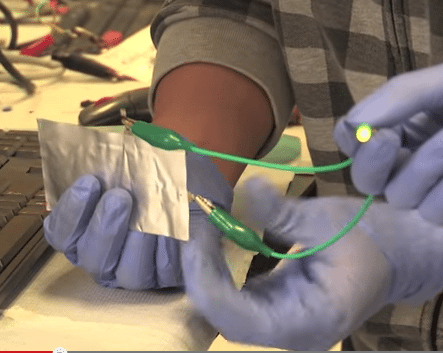
An accident may have led to a discovery that could change the way we use our smartphones. Researchers at Stanford University said they inadvertently discovered that graphite can be used with aluminum to create a new battery that will charge fully in as little as one minute.
The battery uses a negatively charged anode made of aluminum and a positively charged cathode made of graphite. It produces 2 volts of electricity, about half the voltage in an average smartphone battery. By using two of the batteries and a converter, the researchers were able to produce 5 volts, more than enough to power a smartphone.
Smartphones on the market today use batteries that produce around 4 volts of electricity. The iPhone 6, for example, has a 3.8 volt battery. Battery life is a key metric for smartphone buyers, and this was not directly addressed by the researchers.
In a video published on YouTube, the researchers show the battery powering an LED light and then they show two batteries powering a smartphone. They do not demonstrate the ultra fast charging capability of the battery.
Because aluminum is inexpensive relative to many other materials, scientists have long been interested in creating an aluminum battery. Stanford said that a big problem has been finding materials capable of producing sufficient voltage after repeated cycles of charging and discharging.
Now that problem may have been solved. The research team, led by chemistry professor Hongjie Dai, said its battery was able to withstand more than 7,500 cycles without any loss of capacity. The researchers said the average lithium-ion battery withstands about 1,000 charges.
The team said its battery can bend and therefore has potential for use in flexible electronic devices. It can also be pierced without catching fire, making it safer than lithium-ion batteries.
Aluminum holds less energy per unit than does lithium ion. This lower energy density, combined with the lower voltage, are two major hurdles the battery will probably need to scale before commercialization for smartphones becomes an option. Dai said neither hurdle is insurmountable.
“Improving the cathode material could eventually increase the voltage and energy density,” he said. “Otherwise, our battery has everything else you’d dream that a battery should have: inexpensive electrodes, good safety, high-speed charging, flexibility and long cycle life. I see this as a new battery in its early days. It’s quite exciting.”